Uncategorized
Are You a Man or a Mouse?
DISCLAIMER and WARNING:
I’m an animal lover, through and through. This article covers some particulars of animal research models which are essentially no longer used due to advances in non-invasive means of quantifying muscle growth in humans (e.g., with MRI or ultrasound) not available decades ago. These methods may be disturbing to you, as they were and still are to some degree to me. I hope that, rather than burying the results of these studies for that reason, we may at least make these unique experimental models relevant as we pursue our passion for bodybuilding. Nonetheless, be warned that these animal experiments, while approved the relevant ethical committees in place when carried out, may be disturbing to some.
What if you could subject your muscles to an essentially “unlimited” training stimulus, i.e., where you literally lived in the gym, training, eating and sleeping? Would you be a larger bodybuilder?
Are your muscles capable of growth substantially beyond what you can reasonably generate, even with a program as brutal as Mountain Dog Training? Or do you suspect that your nervous system and willpower exceed the growth capacity of your muscles?
Well, this article will take a look at scientific studies, using a few different “models of muscle growth,” conducted with research animals that can tell us about the grand “what if” scenario: A bodybuilder who can live without the “normal” restrictions of everyday life and be free to impose a truly relentless stimulus for muscle growth. We’ll start with some baseline information: How do scientists meaure muscle growth and how much muscle growth you can expect from a “normal” progressive overload-based weight training regimen?
Muscle Growth: How Much from “Normal” Weight Training?
Muscle size is typically quantified with a muscle biopsy, where muscle fiber cross-sectional area (CSA) is measured, or by measuring whole muscle CSA or volume (using a magnetic resonance imager or MRI) or ultrasound (the same technology in use in hospitals). As expected, the relative increase in muscle volume and fiber size after a weight training regime are in the same ballpark, although larger changes in fiber CSA versus whole muscle CSA or volume have been found(1-3). The same general agreement in size changes, losses in this case, usually holds true for atrophying muscle(4, 5), although small changes in CSA may be more readily detected when the whole muscle is measured(6, 7). Imaging an entire muscle preferable too, simply because it’s not nearly as painful or invasive as removing a muscle biopsy.
Using these methods, how much muscle growth can one expect from a weight training regime? Starting out as an untrained newbie, the upper end of increases of CSA after 4-5 months of a progressively-overloaded weight training program would range from ~20 to less than 50%(8, 9). In a study of mine from “back in the day” where only one quad was trained for 8 weeks, just a ~10% increase in quadriceps CSA left many of my beloved subjects feeling a bit like fiddler crabs, due to the highly visible enlargement of the trained quad. We often had a good chuckle immediately after training sessions as we rounded out our 8 weeks together, when they would hobble out of the lab with a giant pump only in their already noticeably overgrown quad(10).
Competitive powerlifters and bodybuilders who have been pounding away in the gym for years have fiber and muscle sizes that range from 30 – 90% larger than sedentary controls(11-14). One notable study found that fibers of the calf muscles of exceptional elite athletes may be as much as 2.5 times as large as those of your typical (completely sedentary) couch potato(15). (Note here that long-term training studies, from newbie to advanced bodybuilder, are sorely lacking. Although a topic perhaps for another article here at mountaindogdiet.com, large competitive bodybuilders have also been found to have muscle fibers of relatively normal sizes(14). This could mean that that their large muscle size is the result of a genetic endowment with a large number of fibers (simply enlarged to normal sizes with training(16)) and/or that hyperplasia (an increase in fiber number) is also at work(17).
Reality Check: Real Muscle
Over the course of a bodybuilder’s career, a gain of 50-60lb of “stage weight” might be considered successful accumulation of muscle mass, in my opinion. IFBB Pro David Henry competed as a lightweight early in his career (18). I personally was about 160lb on stage about 15 years ago and, considering improved conditioning of my more recent showings, have probably added about 60lb (~27kg) of “stage” muscle over that time. Assuming I would have been close in composition to the scientific standard “reference man” (weighing 154lb with 32 kg of muscle mass) as an untrained adult, given I was a massive 160lb as a high school senior even after several years of training for sports, this constitutes less than a 90% increase in muscle mass(19) (or less than 10% / yr) over my lifting “career.” (I grew fairly steadily by adding roughly 5lb each competitive season, creeping up slowly from the middles into the heavyweight class. Needless to say, I’m no Phil Heath, but I hope gives you an idea of the relative scope of gains that I’ve found to be fairly typical amongst competitive bodybuilders who devote many years to their passion.)
What Happens if You Turn a Rat into a Meathead?
If you’ve spent much time out and about in “God’s Country” or simply watched your fair share of National Geographic or Animal Planet on television, you may share my opinion that, despite some of our miraculous physical feats, humans are probably closer to the bottom of totem pole of athleticism in the Animal Kingdom than we like to admit. I mention this potential bias now because below I will be presenting some extraordinary rates of muscle growth demonstrated in research with rodents and birds. If these findings are simply a matter of those animals adapting “better” (with more growth) to resistive overload than humans, then they may not have relevance to us as bodybuilders. (We’d be comparing apples to oranges, and in essence, these data might just tell us that rats make better bodybuilders than men.)
Actually, research rats have indeed been turned into “gym rats,” so we have some general idea how well these animals’ muscle grow in comparison to people, given similar stimuli. Lab rats taught to climb vertically for food reward (5x / week, with loads progressing to over >150% of animal body weight) increased overall muscle weight by 8-9% over two months, increasing type II fiber size about 16%(20). Similarly, rats trained to “tip-toe squat” with up to 130% of body mass increased plantaris muscle mass (one of the calf muscles) by over 30% in 8 weeks (compared to growing control animals)(21, 22). Somewhat less impressive were the “gains” experienced by cats who, for a food reward, performed progressively overloaded “wrist curls” almost daily for training periods lasting ~6 mo, 2yr and 3 years, which increased muscle mass by only 6, 16 and 24%, respectively(23-25).
Interestingly, a calf weight training regime (5 sessions / week) where contractions were evoked using electrical stimulation (animals were unconscious during training) increased plantar flexor (“calf”) muscle weight by approximately 13-18% over 16 weeks(26). In comparison, humans who volunteered to endure 8 weeks of quad muscle electrical stimulation (only twice / week) experienced CSA gains of 10-11% in just half the time (8 weeks) and less than 20% as many sessions (only twice weekly)(10, 27).
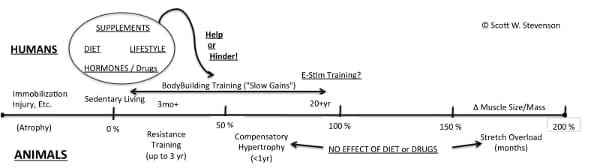
All in all, although the data are sparse, these animal data fall roughly within the range of muscle growth found in humans doing weight training.
Don’t Try This at Home
It’s worth noting that the electrical stimulation-evoked quadriceps training mentioned above(10, 27), conducted using an isokinetic dynamometer, actually evokes twice the rate of muscle growth compared when subjects train under their own volition(27). By using electrical stimulation units, in this case quite powerful machines available only with medical license or for research purposes (DON’T try this at home or your home gym), a large muscle mass can be activated directly via the motor nerves, which bypasses normal reflexive inhibition(28). This means that when using an isokinetic dynamometer (also a expensive piece of equipment), which precisely controls movement speed by a motor capable of more torque than any human, the eccentric / lowering actions occur against tremendous load(29). The stimulated contractions keep the contracting muscle “on” continuously, which actually produces forces greater than what are even possible during maximum voluntary eccentric efforts (unpublished observations). These “supraphysiological” forces during eccentric contractions likely explains why this form of training is so effective(30) and perhaps why it was not enhanced by creatine supplementation(10).
All Day, Baby! All Day! How Fast can Muscle Grow?
So, from the limited “gym rat” information available, humans and mammals respond relatively similarly to a resistance training regimes over the short term, and longer studies (in cats in particular) suggest that, under “normal circumstances,” it may take years to increase muscle mass over 50% beyond the untrained state. More simply, it appears that rats and cats aren’t (clearly) our genetic superior when it comes to packing on freaky muscle size, at least when it comes to pumping iron to do so.
Compensate!
“Force that muscle to grow!” I think I’ve probably muttered that a time or two over the years and definitely heard someone yelling it from across the gym. However, what if you actually gave a muscle no choice but to “adapt or die,” so to speak? How much muscle growth would be possible if a loading stimulus was literally presented to a muscle “All Day (Baby)?”
Well, the “compensatory hypertrophy” model, which may also increase fiber number(31, 32) essentially does just that. The compensatory hypertrophy procedure is carried out by removing the gastrocnemius and sometimes the soleus (calf muscles)(33, 34) of just one leg. This leaves the plantaris (and possibly soleus) in place to take over the load for locomotion during the day. After surgery, rodents regain normal activity levels within a few days(35), and increases in muscle size and protein metabolism appear even within 48 hours(34, 36).
As you might imagine, the phenomenal stress imposed on the muscle left to handle the load (the plantaris and/or soleus) of its missing contractile brethren, leads to substantial inflammation and fluid accumulation initially(37). However, in less than a week, the growing muscle makes substantial gains in strength and endurance(36). After 10 weeks of handling the load of plantar flexion, a rat’s plantaris can be 70% more massive(37). This supersized muscle displays by a tremendous re-organization of blood supply and even markers of neurogenesis(37), perhaps suggesting innervation of newly developing fibers(38). After seven months of compensating, the overloaded soleus of mice is 50% larger(39), roughly equaling my lifetime achievement in the calf department. Interestingly, in a study with mice(39) fiber diameters were only ~10% larger in the central belly of the soleus, and actually smaller nearer the Achilles tendon, in a muscle that had grown to be 50% more massive. Logically, some have suggested implies muscle fiber hyperplasia(17), given the quantitiative disparity in fiber size and muscle mass increases. From a bodybuilding perspective, preferential growth in the center of a muscle belly might even mean that, at least in the case of mice, one can indeed change the shape of a muscle. When it comes to muscle peaks, maybe being a mouse is better than being a man?
Training Trumps All?
The stimulus of the compensatory hypertrophy model is actually so powerful that even without a pituitary gland, the master endocrine gland, i.e., without the effects testosterone, growth hormone and thyroid hormone (and several other hormones), muscle size increases are not dampened(36). Even without those missing hormones and a daily dose of the catabolic corticosteroid cortisone, rat muscles still respond just as well to this super stimulus (at least over the short term)(37, 40). In fact, even after a nearly a week of continuous fasting (no food at all, none of this intermittent fasting stuff), a compensating soleus will beg, borrow and steal amino acids from the blood stream to manage just as much growth as ever (relative to the overall loss of muscle), and still end up larger than the normal soleus of untreated, growing animals consuming a regular diet(36). Even (diabetic) rats who lack the beloved anabolic, anti-catabolic, muscle packing hormone insulin(41) will adapt to compensatory hypertrophy as well a non-diabetic rats(36, 42). And, in case you’re wondering, administering an anabolic androgenic steroid (AAS; nandrolone) also fails to further muscle growth during compensatory hypertrophy(43).
 Super-Duper Stimulus: Stretch overload
Super-Duper Stimulus: Stretch overload
Does this mean that the fastest way to a massive, ripped physique is to spend your days as a fasting, diabetic, hypogonadal, hypothyroid maniac doing high volume, full body workouts? I doubt it, and I’ll come back to this point below. However, to further emphasize the tremendous capacity for adaptation (aka plasticity) of skeletal muscle, an avian (bird) research model called “stretch overload” has demonstrated up to 200% increases in muscle mass (three times normal size) in just a matter of weeks(44). Stretch overload entails adding a progressively heavier weight to the bird’s wing, which produces both an increase in fiber size and fiber number(17, 31, 32). As above, this stimulus is so pronounced that neither of these adaptations are improved upon by administering anabolic steroids(45).
So What Are We Left With?
It seems that “weight training” rodents and cats grow muscle about as well as humans over the short and long haul. This lends credence to the suggestion that the tremendous muscle growth rates of “compensatory hypertrophy” model inform us about what’s possible in terms of human muscle mass accrual, i.e., that more muscle is at least possible above and beyond a what “normal” resistance training can produce. (Avian stretch overload adaptations further underscore the almost ridiculous ability of animal muscle to grow when really put to the test.) Indeed, the stimulus of compensatory hypertrophy is so powerful that neither removal of normal endogenous hormones (growth hormone, insulin, etc.) or food, nor administration of catabolic (cortisol) or anabolic (AAS) hormones substantially affect the growth response(36). That’s powerful stuff right there.
Naturally, 1this is not to say that training parameters, diet, dietary supplement use or even pharmacological strategies have no effect on muscle size. Of course, they do. Volumes of scientific and empirical evidence suggests that diet(46), supplements(47) and drugs(48) can indeed modify muscle mass accumulation in conjunction with weight training. However, this does suggest that skeletal muscle has an adaptive capacity far beyond what bodybuilders typically tap into. The super potent forms of “training” presented here go far beyond a normal bodybuilding routine: They create loading throughout the day, every day (compensatory hypertrophy and stretch overload) or, in the case of electrically-evoked “involuntary” contractions, create “supraphysiological” loading that is practically impossible under normal conditions.
Scott W. Stevenson, PhD, LAc
References
1. Harber, M.P., et al., Aerobic exercise training improves whole muscle and single myofiber size and function in older women. American journal of physiology. Regulatory, integrative and comparative physiology, 2009. 297(5): p. R1452-9. https://www.ncbi.nlm.nih.gov/pubmed/19692660
2. Sanchis-Moysi, J., et al., The upper extremity of the professional tennis player: muscle volumes, fiber-type distribution and muscle strength. Scandinavian journal of medicine & science in sports, 2010. 20(3): p. 524-34. https://www.ncbi.nlm.nih.gov/pubmed/19602193
3. Vissing, K., et al., Muscle adaptations to plyometric vs. resistance training in untrained young men. Journal of strength and conditioning research / National Strength & Conditioning Association, 2008. 22(6): p. 1799-810. https://www.ncbi.nlm.nih.gov/pubmed/18978625
4. Hather, B.M., et al., Skeletal muscle responses to lower limb suspension in humans. Journal of applied physiology, 1992. 72(4): p. 1493-8. https://www.ncbi.nlm.nih.gov/pubmed/1534323
5. Yasuda, N., et al., Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. Journal of applied physiology, 2005. 99(3): p. 1085-92. https://www.ncbi.nlm.nih.gov/pubmed/15860685
6. Lange, K.H., et al., GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. The Journal of clinical endocrinology and metabolism, 2002. 87(2): p. 513-23. https://www.ncbi.nlm.nih.gov/pubmed/11836279
7. Kaser, L., et al., Active therapy for chronic low back pain: part 2. Effects on paraspinal muscle cross-sectional area, fiber type size, and distribution. Spine, 2001. 26(8): p. 909-19. https://www.ncbi.nlm.nih.gov/pubmed/11317113
8. Cureton, K.J., et al., Muscle hypertrophy in men and women. Medicine and science in sports and exercise, 1988. 20(4): p. 338-44. https://www.ncbi.nlm.nih.gov/pubmed/3173042
9. Staron, R.S., et al., Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. European journal of applied physiology and occupational physiology, 1990. 60(1): p. 71-9. https://www.ncbi.nlm.nih.gov/pubmed/2311599
10. Stevenson, S.W. and G.A. Dudley, Dietary creatine supplementation and muscular adaptation to resistive overload. Medicine and science in sports and exercise, 2001. 33(8): p. 1304-10. https://www.ncbi.nlm.nih.gov/pubmed/11474331
11. Prince, F.P., et al., Human muscle fiber types in power lifters, distance runners and untrained subjects. Pflugers Archiv : European journal of physiology, 1976. 363(1): p. 19-26. https://www.ncbi.nlm.nih.gov/pubmed/131933
12. Sale, D.G., et al., Voluntary strength and muscle characteristics in untrained men and women and male bodybuilders. Journal of applied physiology, 1987. 62(5): p. 1786-93. https://www.ncbi.nlm.nih.gov/pubmed/3597252
13. Alway, S.E., et al., Effects of resistance training on elbow flexors of highly competitive bodybuilders. Journal of applied physiology, 1992. 72(4): p. 1512-21. https://www.ncbi.nlm.nih.gov/pubmed/1592744
14. MacDougall, J.D., et al., Muscle fiber number in biceps brachii in bodybuilders and control subjects. Journal of applied physiology: respiratory, environmental and exercise physiology, 1984. 57(5): p. 1399-403. https://www.ncbi.nlm.nih.gov/pubmed/6520032
15. Alway, S.E., et al., Functional and structural adaptations in skeletal muscle of trained athletes. Journal of applied physiology, 1988. 64(3): p. 1114-20. https://www.ncbi.nlm.nih.gov/pubmed/3366734
16. MacDougall, J.D., et al., Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. European journal of applied physiology and occupational physiology, 1982. 48(1): p. 117-26. https://www.ncbi.nlm.nih.gov/pubmed/7199447
17. Antonio, J. and W.J. Gonyea, Skeletal muscle fiber hyperplasia. Medicine and science in sports and exercise, 1993. 25(12): p. 1333-45. https://www.ncbi.nlm.nih.gov/pubmed/8107539
18. Henry, D.B. TooPumped.net. 2013 [Accessed 4/20/2013]; (https://toopumped.net/contesthistory.html.).
19. Snyder, W.S., et al., Gross and Elemental Content of Reference Man, in Report of the Task Group on Reference Man. 1975, Pergamon Press: Oxford. p. 273-285.
20. Yarasheski, K.E., et al., Effect of heavy resistance training on skeletal muscle hypertrophy in rats. Med Sci Sports Exerc, 1987. 19(2): p. S15.
21. Klitgaard, H., A model for quantitative strength training of hindlimb muscles of the rat. Journal of applied physiology, 1988. 64(4): p. 1740-5. https://www.ncbi.nlm.nih.gov/pubmed/3379005
22. Ho, K.W., et al., Skeletal muscle fiber splitting with weight-lifting exercise in rats. The American journal of anatomy, 1980. 157(4): p. 433-40. https://www.ncbi.nlm.nih.gov/pubmed/7405877
23. Mikesky,
A.E., et al., Changes in muscle fiber size and composition in response to heavy-resistance exercise. Medicine and science in sports and exercise, 1991. 23(9): p. 1042-9. https://www.ncbi.nlm.nih.gov/pubmed/1943624
24. Gonyea, W., et al., Skeletal muscle fiber splitting induced by weight-lifting exercise in cats. Acta physiologica Scandinavica, 1977. 99(1): p. 105-9. https://www.ncbi.nlm.nih.gov/pubmed/139079
25. Gonyea, W.J., Role of exercise in inducing increases in skeletal muscle fiber number. Journal of applied physiology: respiratory, environmental and exercise physiology, 1980. 48(3): p. 421-6. https://www.ncbi.nlm.nih.gov/pubmed/7372511
26. Wong, T.S. and F.W. Booth, Skeletal muscle enlargement with weight-lifting exercise by rats. Journal of applied physiology, 1988. 65(2): p. 950-4. https://www.ncbi.nlm.nih.gov/pubmed/2459101
27. Ruther, C.L., et al., Hypertrophy, resistance training, and the nature of skeletal muscle activation. J.Strength Cond.Res., 1995. 9: p. 155-159.
28. Binder-Macleod,
S.A. and L. Snyder-Mackler, Muscle fatigue: clinical implications for fatigue assessment and neuromuscular electrical stimulation. Physical therapy, 1993. 73(12): p. 902-10. https://www.ncbi.nlm.nih.gov/pubmed/8248298
29. Dudley, G.A., et al., Effect of voluntary vs. artificial activation on the relationship of muscle torque to speed. Journal of applied physiology, 1990. 69(6): p. 2215-21. https://www.ncbi.nlm.nih.gov/pubmed/2077019
30. Dudley, G.A., et al., Importance of eccentric actions in performance adaptations to resistance training. Aviation, space, and environmental medicine, 1991. 62(6): p. 543-50. https://www.ncbi.nlm.nih.gov/pubmed/1859341
31. Taylor, N.A. and J.G. Wilkinson, Exercise-induced skeletal muscle growth. Hypertrophy or hyperplasia? Sports medicine, 1986. 3(3): p. 190-200. https://www.ncbi.nlm.nih.gov/pubmed/3520748
32. Kelley, G., Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. Journal of applied physiology, 1996. 81(4): p. 1584-8. https://www.ncbi.nlm.nih.gov/pubmed/8904572
33. Lieber, R.L., Skeletal muscle adapation to increase use, in Skeletal muscle structure and function : implications for rehabilitation and sports medicine. 1992, Williams & Wilkins: Baltimore. p. ix, 303 p.
34. Goldberg, A.L., Mechanisms of growth and atrophy of skeletal muscle. Muscle biology, 1972. 1: p. 89-118. https://www.ncbi.nlm.nih.gov/pubmed/4578602
35. Goldberg, A.L., Work-induced growth of skeletal muscle in normal and hypophysectomized rats. The American journal of physiology, 1967. 213(5): p. 1193-8. https://www.ncbi.nlm.nih.gov/pubmed/6054866
36. Goldberg, A.L., et al., Mechanism of work-induced hypertrophy of skeletal muscle. Medicine and science in sports, 1975. 7(3): p. 185-98. https://www.ncbi.nlm.nih.gov/pubmed/128681
37. Tamaki, T., et al., Multiple stimulations for muscle-nerve-blood vessel unit in compensatory hypertrophied skeletal muscle of rat surgical ablation model. Histochemistry and cell biology, 2009. 132(1): p. 59-70. https://www.ncbi.nlm.nih.gov/pubmed/19322581
https://link.springer.com/article/10.1007%2Fs00418-009-0585-1
38. Tamaki, T. and S. Uchiyama, Absolute and relative growth of rat skeletal muscle. Physiology & behavior, 1995. 57(5): p. 913-9. https://www.ncbi.nlm.nih.gov/pubmed/7610144
39. Vaughan, H.S. and G. Goldspink, Fibre number and fibre size in a surgically overloaded muscle. Journal of anatomy, 1979. 129(Pt 2): p. 293-303. https://www.ncbi.nlm.nih.gov/pubmed/500488
40. Kurowski, T.T., et al., Countereffects of compensatory overload and glucocorticoids in skeletal muscle: androgen and glucocorticoid cytosol receptor binding. Journal of steroid biochemistry, 1984. 21(2): p. 137-45. https://www.ncbi.nlm.nih.gov/pubmed/6332946
41. Fulks, R.M., et al., Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. The Journal of biological chemistry, 1975. 250(1): p. 290-8. https://www.ncbi.nlm.nih.gov/pubmed/1141208
42. Goldberg, A.L., Role of insulin in work-induced growth of skeletal muscle. Endocrinology, 1968. 83(5): p. 1071-3. https://www.ncbi.nlm.nih.gov/pubmed/5685568
43. Tamaki, T., et al., Anabolic-androgenic steroid does not enhance compensatory muscle hypertrophy but significantly diminish muscle damages in the rat surgical ablation model. Histochemistry and cell biology, 2009. 132(1): p. 71-81. https://www.ncbi.nlm.nih.gov/pubmed/19319558
44. Timson, B.F., Evaluation of animal models for the study of exercise-induced muscle enlargement. Journal of applied physiology, 1990. 69(6): p. 1935-45. https://www.ncbi.nlm.nih.gov/pubmed/2076987
45. Alway, S.E. and L.A. Starkweather, Effect of anabolic steroids on new fiber formation and fiber area during stretch-overload. Journal of applied physiology, 1993. 74(2): p. 832-7. https://www.ncbi.nlm.nih.gov/pubmed/8458803
46. Churchward-Venne, T.A., et al., Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutrition & metabolism, 2012. 9(1): p. 40. https://www.nutritionandmetabolism.com/content/9/1/40
47. Kreider, R.B., Dietary supplements and the promotion of muscle growth with resistance exercise. Sports medicine, 1999. 27(2): p. 97-110. https://www.ncbi.nlm.nih.gov/pubmed/10091274
48. American College of Sports Medicine, Position statement on the use of anabolic-androgenic steroids in sports. Medicine and science in sports, 1987. 19(5): p. 534-539. https://journals.lww.com/acsm-msse/Citation/1987/10000/Position_Stand_on_The_Use_of_Anabolic_Androgenic.23.aspx




